How Oncoshot Built a Federated Data Platform on AWS to Accelerate Clinical Trials
Ruslan Enikeev, Andrey Gonostarev, Diane Galistan, Hendrik Chan, Shanicus Yee, and Noorbakht Khan, 19 March 2024
While patient-facing organizations (such as clinics, hospitals, and laboratories) understand the importance of collecting and managing patient data, there is currently no efficient framework for sharing this data securely.
To mitigate risks, many organizations opt to retain patient data rather than risk its exposure. Consequently, patient data becomes siloed within these organizations, inaccessible to the industry at large.
At the same time, non-patient-facing organizations (such as academic and contract research groups) require access to patient population information for the purposes of research and drug discovery. However, they often lack the infrastructure to securely exchange data with other healthcare institutions.
This dynamic presents a challenge to clinical trial efficiency. Delays in the development of potentially life-saving treatments can have profound implications, particularly for patients grappling with serious or life-threatening conditions. These individuals often find themselves in a race against time, fervently hoping for access to new treatment options that could offer a lifeline in their battle against illness.
In this post, we describe how Oncoshot built a federated data platform on Amazon Web Services (AWS) to enable secure data exchange and connect industry experts with crucial insights derived from real-time patient data. These insights inform decision-making, lead to accelerated patient enrollment into clinical trials, and reduce drug development costs. Ultimately, Oncoshot expedites the timely delivery of potentially life-saving therapies to patients.
Oncoshot is an AWS Partner that provides innovative real-time health insights exchange, serving as a bridge between healthcare providers and the healthcare industry.
Key Challenges
For many patients, clinical trials represent a beacon of hope that offers a chance for improved outcomes or even a potential cure. Consequently, any delays in finding matching trials not only prolong their suffering but also dampen the optimism and enthusiasm surrounding their treatment journey.
The financial implications of trial delays are equally substantial. Research indicates that 80% of trials face delays of at least a month, resulting in potential losses of approximately $600,000 per day. In more severe cases, particularly for complex trials or those involving large patient cohorts, these losses can skyrocket to an astounding $8 million daily.
Such financial implications underscore the urgent need for streamlined processes and secure data-sharing frameworks within the clinical trial landscape. Swift and efficient trial execution has significant benefits for patients in need of innovative treatments and ensures the sustainability and progress of the broader healthcare industry.
The clinical trials process is commonly faced with several key challenges, including:
Patient recruitment and retention; trial delays and costs: Finding and enrolling suitable participants within a specific timeframe can be difficult. Low participation rates and high dropout rates can lead to delays and compromised trial results.
Data quality and integrity: Ensuring accurate data collection, recording, and reporting is vital. Poor data quality can impact the validity of trial results and regulatory approval.
Resource constraints: Clinical trials require substantial financial, human, and infrastructure resources. Limited funding and lack of skilled personnel can hinder the smooth progress of trials.
Geographical dispersion: Multi-site trials can face challenges in coordinating activities and ensuring consistency across different sites.
Patient diversity: Ensuring diverse participant representation is important for the applicability of trial results to broader populations. Recruitment of diverse patients can be challenging.
Stakeholder collaboration: Effective collaboration between various stakeholders, including sponsors, researchers, clinicians, and patients, is necessary for successful trial execution.
Solution Overview
At its inception, the Oncoshot platform took the form of a conventional centralized business-to-consumer (B2C) software as a service. It operated through a single web application and maintained a central database for patient data storage.
In its initial SaaS version, individual patients would upload their profiles to the Oncoshot platform, which would employ a patient-to-trial matching technology to assist in finding suitable matches with existing trials.
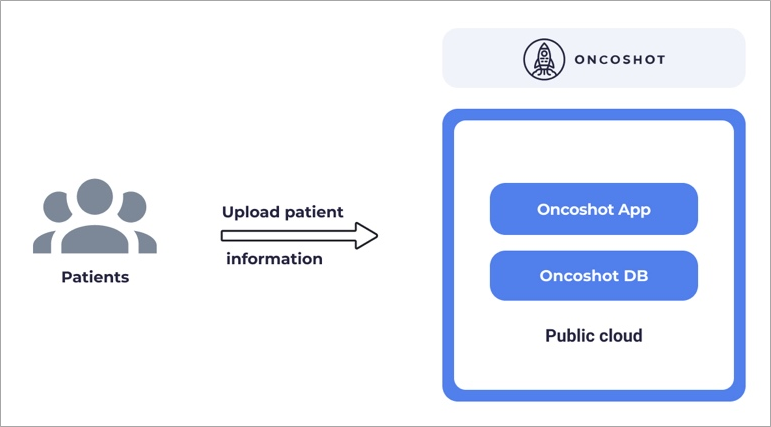
Figure 1 – Original architecture of the Oncoshot platform.
Federated Architecture for Hospitals
Due to Oncoshot’s initial emphasis on individual patients and caregivers, this straightforward and centralized solution was the fitting choice. Yet, over time the patient-to-trial matching technology began to draw attention from hospitals and clinics. The prospect of efficiently scanning entire patient pools to identify eligible candidates for trials sparked interest, prompting discussions about onboarding the first batch of hospitals onto the platform.
As the focus shifted towards larger hospitals, a centralized solution proved unfeasible. Despite Oncoshot exclusively handling de-identified patient data, hospitals insisted on heightened security measures. Systems that handle patient data in the healthcare sector, such as electronic medical records (EMRs), are typically deployed within a hospital’s infrastructure. Data are retained within the environment rather than being exposed externally.
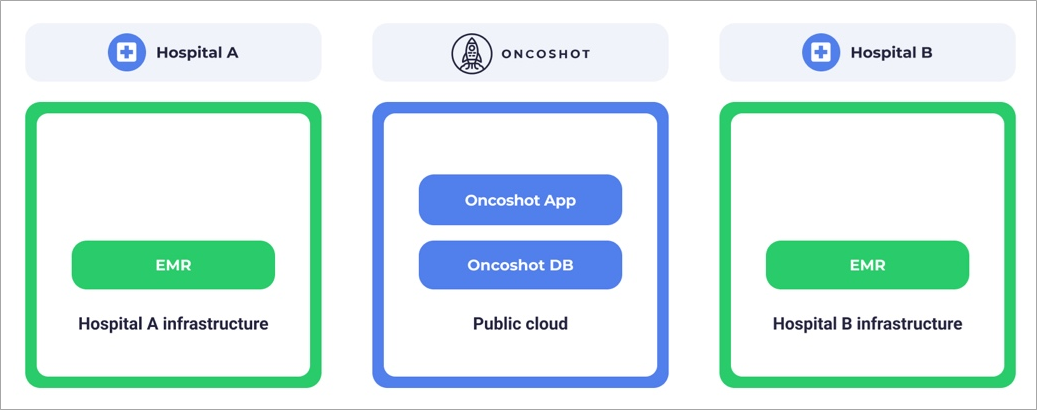
Figure 2 – Patients’ data reside in hospital’s infrastructure.
The problem could be summarized into the question: How can we apply a matching algorithm using data from multiple healthcare institutions without data being directly shared ?” A comparable challenge had also emerged in the field of machine learning and the solution to the problem, known as federated machine learning, hinged on a straightforward concept: if the data can’t come to the software, the software must go to the data.
Inspired by this, Oncoshot adopted a similar approach, bringing the matching algorithm to the hospitals through a federated architecture.
While individual patient data remains within a hospital’s confines, the Oncoshot platform generates aggregated patient population insights that interact with the broader healthcare landscape. This distinctive feature marks a significant departure from traditional siloed approaches to patient data.
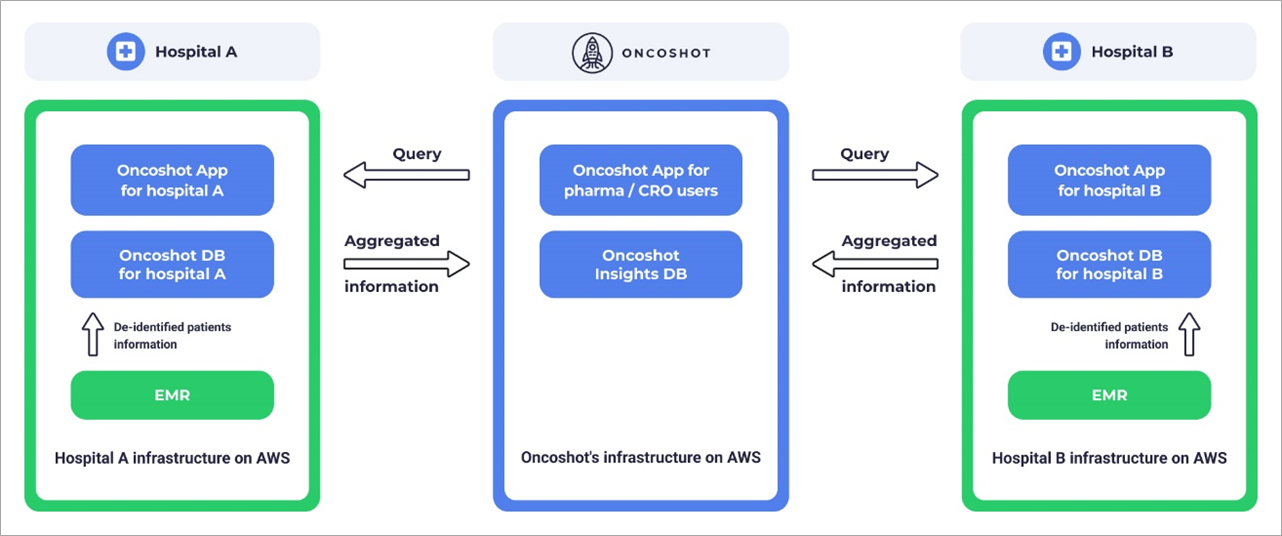
Figure 3 – Federated architecture for hospitals.
In the federated architecture approach, the Oncoshot platform comprises of federated units securely deployed within hospital alongside a central cluster tailored for pharma or contract research organization (CRO) users.
The federated units are identical pieces of infrastructure running in a hospital’s environment. Oncoshot uses infrastructure as code (IaC) to ensure consistent and fast deployment of units in each hospital. Each federated unit includes a Kubernetes cluster running on Amazon Elastic Kubernetes Service (Amazon EKS) and a PostgreSQL database running on Amazon Relational Database Service (Amazon RDS).
Oncoshot federated units are currently deployed in hospitals in multiple countries across the Asia Pacific and Japan (APJ) region and a central cluster for pharma/CRO users. Given this application does not retain any patient data, it does not necessitate federalization.
The diagram below illustrates the components deployed within a hospital’s AWS environment.
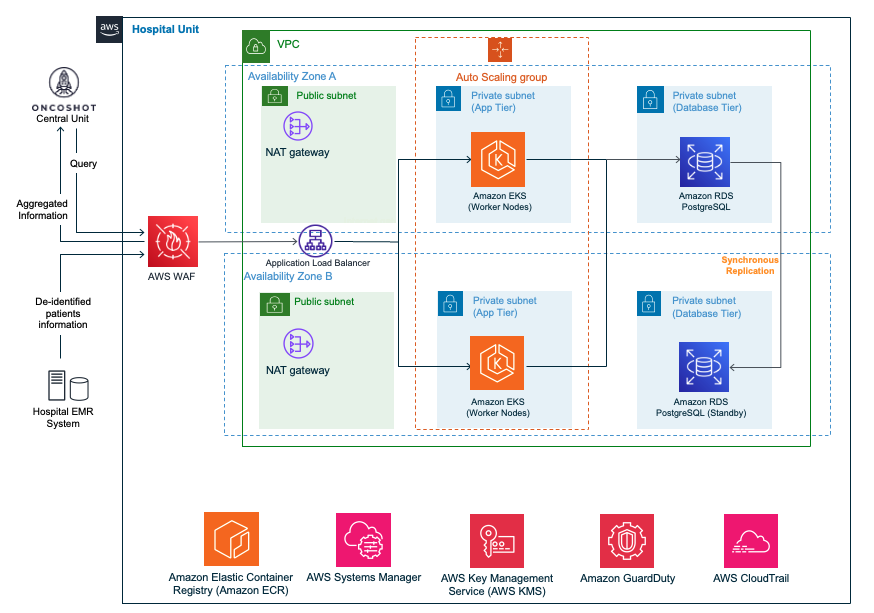
Figure 4 – Oncoshot federated unit deployed in a hospital’s AWS environment.
Generate Matching Insights
Once the federated unit for hospital is deployed, it’s registered with the Oncoshot platform to ensure synchronization with the central unit. This registration process includes implementing stringent security measures to establish a secure connection between the central and hospital units.
Oncoshot holds certifications in various international compliance standards that fully align with the requirements of operating in the HealthTech industry, including ISO 27001, ISO 27017, ISO 27018, and SOC2. It ensures the platform continuously complies with data privacy regulations such as GDPR, PDPA, and HIPAA.
De-identified patient information is transmitted securely from internal hospital systems into the Oncoshot federated unit. Once this hospital onboarding process is finalized, pharma/CRO users (sponsor users) of the central Oncoshot unit can generate queries based on the eligibility criteria outlined in the study protocol. These queries are distributed to all registered hospital units.
When hospital accepts a query, a local matching algorithm running in the federated unit calculates the eligibility of its patients for the study. The outcome of this calculation is referred to as a matching insight. Details regarding the matching insight from each participating hospital are relayed back to the central unit, where they are aggregated once more and subsequently presented to the sponsor user. Watch this two-minute video to better understand this process.
Sponsor users apply these insights in decision making, such as determining which countries or trial sites best fit the requirements of their clinical trials. This reduces the wait time for feasibility to site activation, potentially cutting it down from 3-6 months to 1-3 weeks.
It’s crucial to reiterate that throughout this process, all de-identified patient information remains within the hospital’s AWS cloud infrastructure. Only aggregated patient data is shared with sponsor users in real-time. Below is a screenshot showcasing a study’s insights page. Note that the organizations depicted below are fictitious.
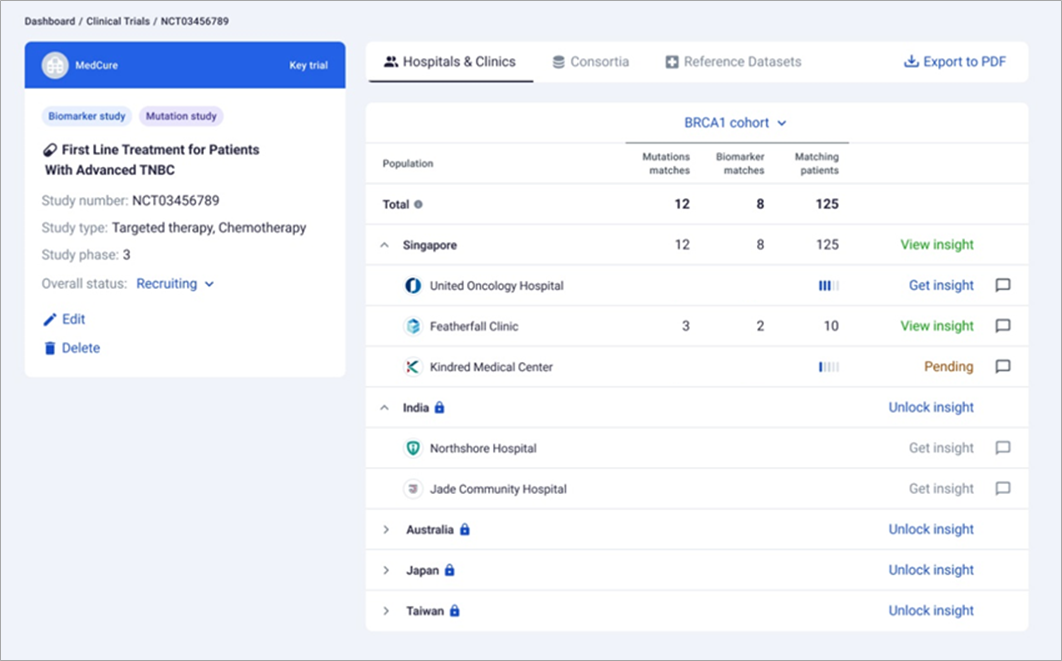
Figure 5 – Matching insights with fictitious data.
Conclusion
The Oncoshot platform stands as a unique solution for the healthcare industry, enabling the exchange of patient population insights without the need to expose or transfer patient data. Oncoshot firmly believes that federated architecture is the future of healthcare.
Are you ready to redefine healthcare collaboration? Join the Oncoshot ecosystem today and tap into the potential of secure data exchange, enriched insights, and innovation. Contact support@oncoshot.com to learn more about joining the Oncoshot ecosystem.
Oncoshot – AWS Partner Spotlight
Oncoshot is an AWS Partner that provides innovative real-time health insights exchange, serving as a bridge between healthcare providers and the healthcare industry.
This article was originally published on AWS.